Clinical
BIOEQUVALENCE STUDY
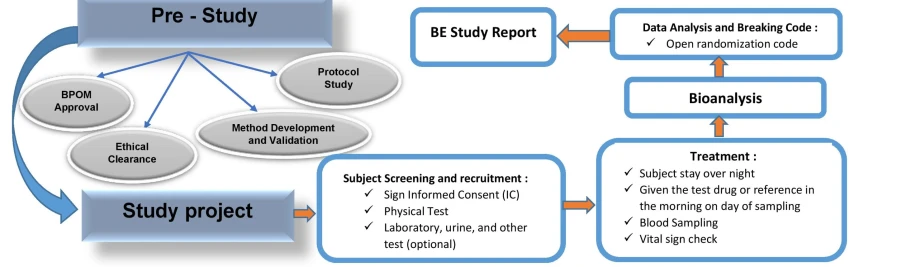
Bioavailability and bioequivalence (BA/BE) studies are required to ensure therapeutic equivalence between a pharmaceutically equivalent test drug and reference drug. Ensuring uniformity in standards of quality, efficacy, and safety of pharmaceutical products.
Omega Research has a BA/BE unit handled by a professional team, our unit offers standardized technology and fast test results. Our research team consists of qualified doctors and paramedics who will monitor BE study subjects throughout the study.

